Blog Post: Exploring New Microfabrication Services on the Goodfellow Podcast
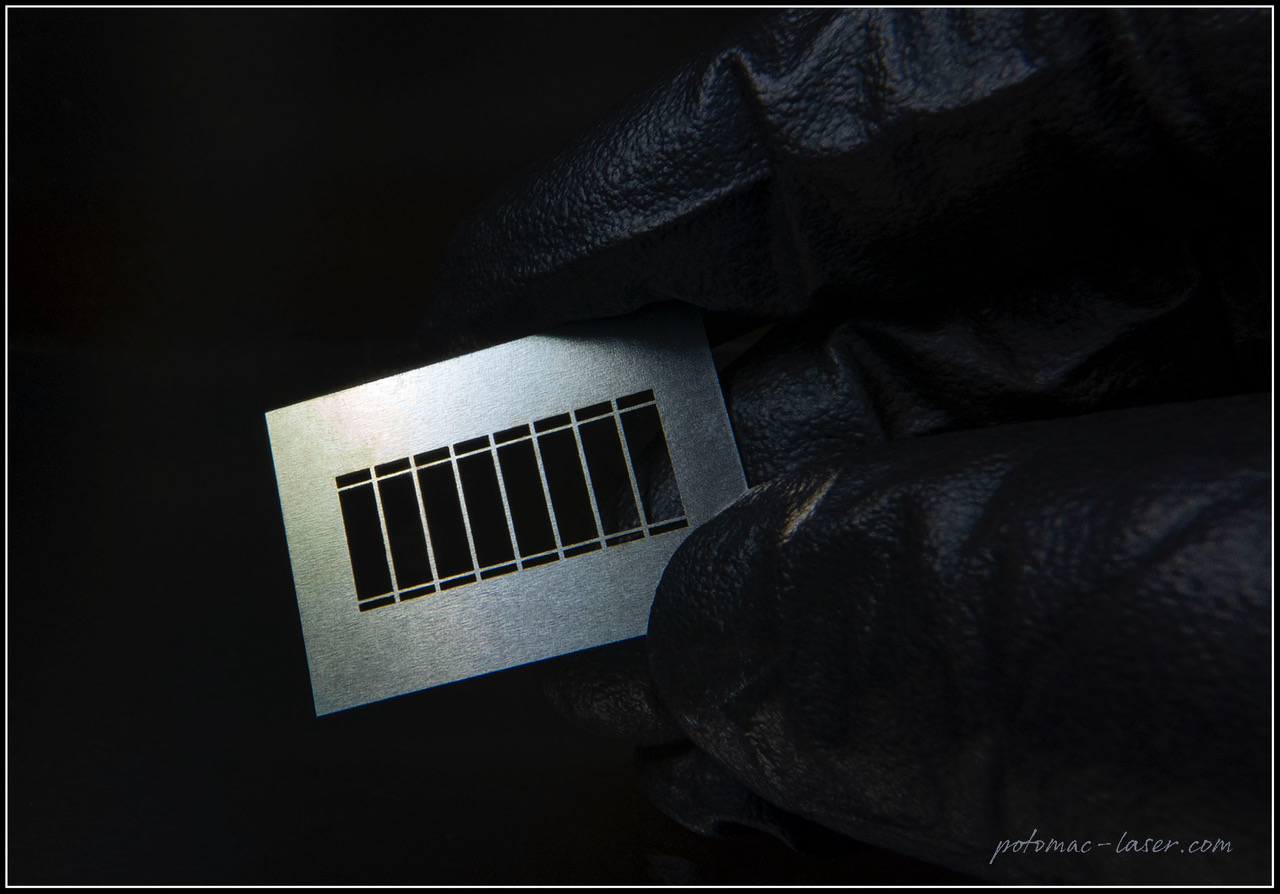
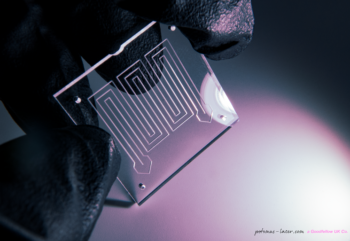
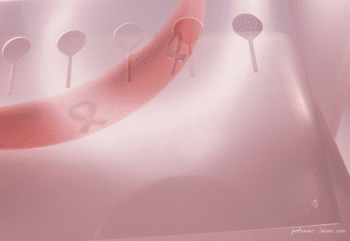
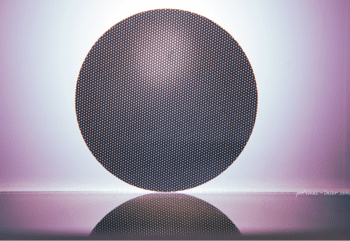
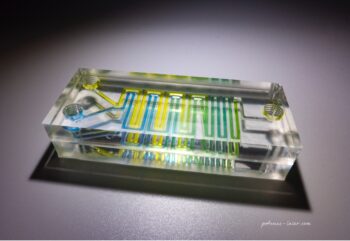
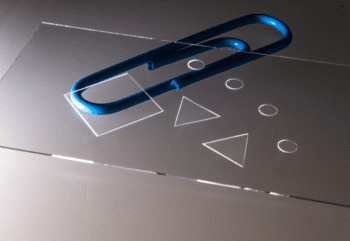
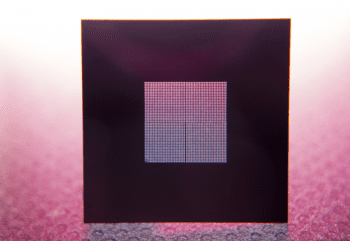
Episode #13 – Microfabrication: The New Services Available from Goodfellow https://www.goodfellow.com/usa/resources/ep-13-microfabrication-services-now-available/ In June 2024, Goodfellow acquired Potomac Photonics, seamlessly integrating their cutting-edge micromanufacturing services into the Goodfellow portfolio. This strategic acquisition expands Goodfellow’s capabilities to include specialized services such as small hole drilling, laser micro welding, micro CNC, and much more. It also marks the...